It may be a group of pigs that infect you with incurable "super bacteria"
Original zi yi Guo ke
Imagine such a scene: a new drug-resistant bacteria will be popular among people in the future, and you are unfortunately infected and lying in the intensive care unit. All the antibiotics injected into your body are completely ineffective, and your life is dying, and you will die like many infected people …
Terrible, isn’t it?
In order to keep yourself alive and avoid the above scene becoming a reality, can you get rid of the habit of taking antibiotics easily from now on to ensure your safety?
No, you don’t abuse antibiotics, your family and friends may abuse them, strangers you meet on the road may abuse them, and even pigs may abuse them.
However, does the use of antibiotics have anything to do with my infection? Of course, drug-resistant bacteria from other people and animals can also infect you.

Abuse of antibiotics by others or animals may also infect you giphy drug-resistant bacteria.
Gonorrhea in the U.S navy is difficult to treat.
Because the sex workers used the wrong antibiotics?
In the 1960s, millions of sailors gathered all the year round at the US Naval Base in subic bay. A large number of people flow has promoted the prosperity and development of local economy, especially bars, nightclubs and brothels. Navy soldiers often hang around these places as long as they are not on duty.
After indulging, many people go to see a military doctor as soon as they return to the boat because they find urethral secretions. They were diagnosed with gonorrhea, that is, gonococcal infection, and received standard penicillin treatment. But strangely, the treatment effect of the navy is not ideal, and the number of infected people is increasing.
The preventive medicine department of the navy conducted an investigation and found that there were two reasons for the failure of the navy’s treatment: one was misdiagnosis, and half of the people suffered from nongonococcal urethritis (such as chlamydia and mycoplasma infection) instead of gonorrhea; The second is drug resistance. The other half of the soldiers who were really infected with gonococcus had strong drug resistance to penicillin, which was not common at that time.
Why is there such a large-scale drug resistance at the naval base in subic bay?
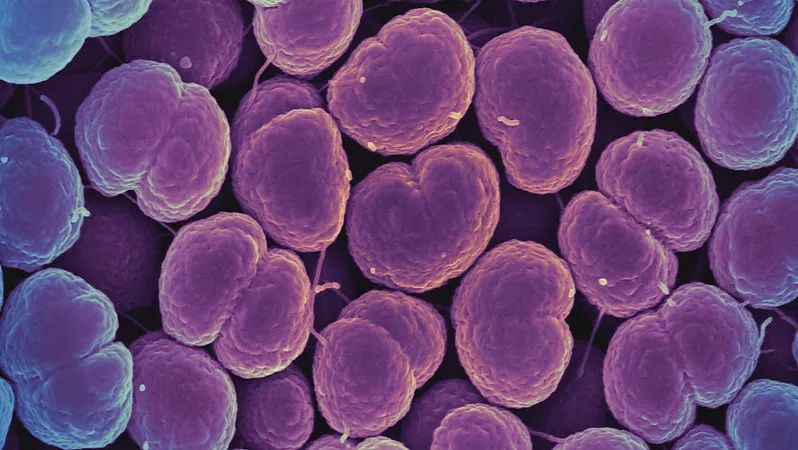
Neisseria gonorrhoeae is resistant to penicillin, which leads to unsatisfactory treatment effect of gonorrhea | flickr
Gonorrhea is mainly transmitted through sexual contact. Considering the common "recreational activities" of the navy, the preventive medicine department began an investigation from sex workers. It turns out that in a clinic where sex workers gather, doctors use benzathine penicillin to treat gonorrhea of sex workers, and they also go to the pharmacy to buy this penicillin themselves.
However, killing Neisseria gonorrhoeae, especially Neisseria gonorrhoeae resistant to penicillin, requires the use of fast-acting drugs, and the drugs cannot stay in the body for too long, otherwise it will provide natural selection opportunities for drug-resistant bacteria. Therefore, the most suitable drug is procaine penicillin, not benzathine penicillin, which has slow onset and long retention time in the body. Excessive use of the same wrong drug has produced penicillin-resistant Neisseria gonorrhoeae in sex workers.
At the same time, the preventive medicine department also found that when the sex workers were examined in this small clinic, the doctor never disinfected the vaginal dilator to improve efficiency, but put a big bucket of water beside him. After each patient was examined, he rinsed it in the bucket and then continued to examine the next one. This irregular operation paved a broad road for the spread of pathogenic bacteria …
In this way, the drug-resistant bacteria spread from one sex worker to another, and then infected the navy soldiers through sexual transmission, and the soldiers then spread them to other sex workers, which led to the widespread spread of drug-resistant bacteria.
After discovering the cause, the Department of Preventive Medicine improved the treatment scheme of gonorrhea, and solved the problem of cross infection by purchasing sterilizers and a large number of vaginal dilators. Finally, the number of patients with drug-resistant gonorrhea plummeted, and the situation of seafarers improved.

Drug-resistant bacteria repeatedly "jump horizontally" among sex workers and naval soldiers, leading to the widespread spread of drug-resistant bacteria | pexels
Pigs eat antibiotics
It can also "upgrade" the bacteria on people.
Drug-resistant bacteria can spread not only between people, but also between people and animals.
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the common multi-drug resistant bacteria, also known as "superbugs".
It is speculated that in 2019, more than 100,000 deaths were related to MRSA. In a study published in June, 2022, Lucy A. Venat of Cambridge University proposed that MRSA (livestock-associated methicillin-resistant Staphylococcus aureus, LA-MRSA) in livestock was an important reason for the increasing human infection with MRSA.
Simply put, if pigs eat more antibiotics, they can also "pass" drug-resistant bacteria to people.
But this "transmission" is not that bacteria directly run from pigs to people, but that drug-resistant bacteria in pigs pass the "skill" of drug resistance to bacteria in people. This is achieved by Mobile genetic elements, MGEs). MGEs can "transport" drug-resistant genes from one bacterium to another, even if the two bacteria belong to different species.
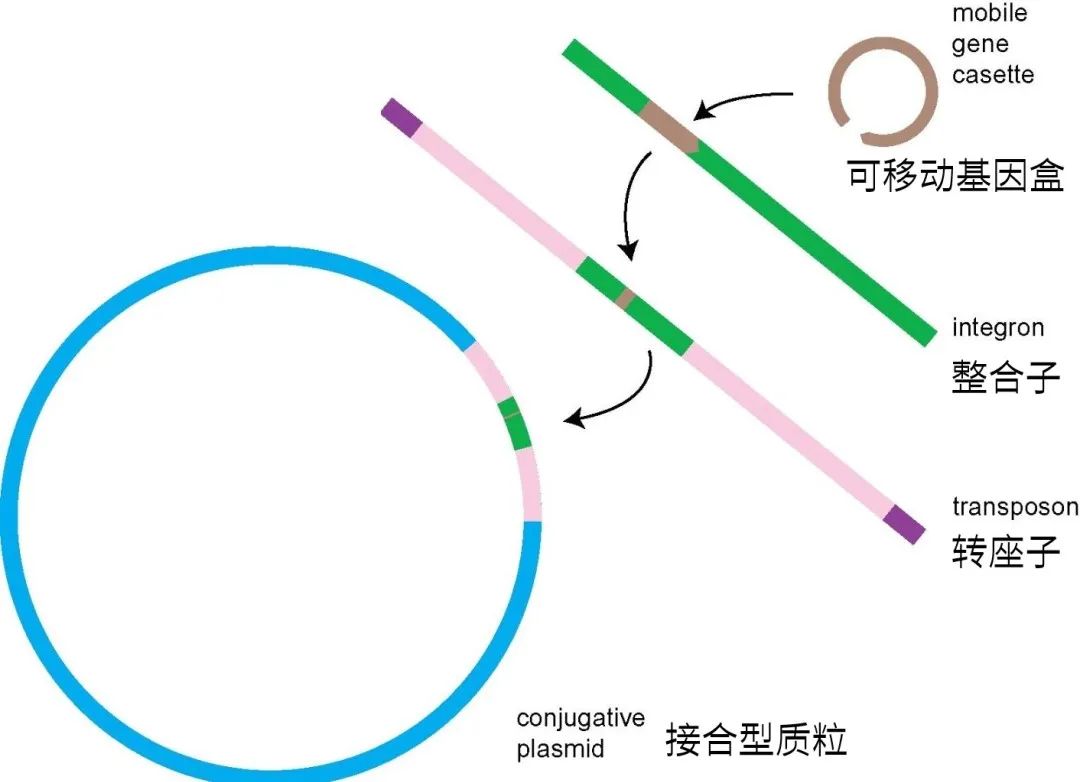
Drug-resistant genes can be integrated into plasmids, and then enter other bacteria through plasmids.
Scientists have found that among the most common LA-MRSA in Europe, one MGEs with drug resistance gene has been stably inherited for 57 years, which dates back to the time when antibiotics were widely used in livestock.
When livestock use a large number of antibiotics to produce LA-MRSA, the related drug-resistant genes can be grafted into human Staphylococcus aureus through MGEs transfer, so that the common Staphylococcus aureus can be "upgraded" to drug-resistant MRSA. Even if humans did not use antibiotics at that time, they would be infected with bacteria that were more difficult to treat.
"One world, one health", the uncontrolled use of antibiotics on animals will eventually affect human beings.

After a large number of animals use antibiotics to produce drug-resistant bacteria, the related drug-resistant genes can "upgrade" the bacteria in people | wallpaperflare
Norwegians eat 24 tons of antibiotics every year.
Norwegian salmon eats 48 tons
Today, more and more people are aware of this potential crisis, and the control system of antibiotic use in aquaculture tends to be perfect, but this was not the case decades ago.
After the emergence of antibiotics, people who breed livestock and poultry found that feeding antibiotics can not only fight against some infectious diseases, but also promote the growth of livestock and poultry, increase the output of agricultural meat products and improve profits.
In the 1960s, many countries, such as Britain, Germany, the Netherlands, and the United States, enacted laws to reduce the use of antibiotics-especially to reduce the use of antibiotics for humans by livestock. However, driven by interests, these policies are either strongly resisted or disobeyed, and it is always difficult to implement them.
But there are also successful examples.
Norway has the highest salmon production in the world, and its quality is among the best.

Orange-yellow fish, fresh Q-bomb, is soft, tender and smooth in one bite, melts in the mouth, and the special aroma spreads in the mouth … Well, it’s a bit off topic | flickr.
In 1980s, Norwegian salmon industry developed on a large scale, which brought a problem, that is, furuncle began to spread in fish on a large scale. In order to protect salmon, Norwegian fishermen began to use antibiotics preventively and added them directly to fish feed. By the end of 1980s, the demand for antibiotics was increasing, and fishermen even used cement mixers to mix them into fish feed.
During that time, Norwegians prescribed an average of 24 tons of antibiotics every year, while salmon consumed 48 tons of antibiotics every year. Salmon used twice as many antibiotics as all Norwegians!
These fish feeds mixed with antibiotics were directly poured into the fishing ground and flowed into the nearby waters, so that high levels of antibiotics appeared in fish thousands of meters away from the fishing ground, and even in birds that feed on fish.
Local TV stations have produced relevant documentaries, calling on the government to issue an antibiotic supervision bill. However, the appeal was strongly resisted by fishing groups, and the documentary producers also received anonymous threat bombs. Documentaries have been banned, but the truth will never be buried. The public is paying more and more attention to the problem of drug resistance, and the fishery is under increasing pressure.
Fortunately, people later developed a salmon vaccine that can prevent furuncle, which can be injected from the abdomen of fish through automated procedures. This method not only reduces the use of antibiotics, but also stabilizes the salmon economy and has been vigorously promoted.
In 1994, vaccination of salmon became a routine operation in Norway, and the use of antibiotics plummeted.
In recent years, countries are trying to limit or stop using antibiotics in aquaculture. China has always advocated the rational and standardized use of antibiotics in livestock and poultry breeding. Announcement No.194 issued by the Ministry of Agriculture and Rural Affairs stated that since New Year’s Day in 2020, the addition of antibiotics to feed in China has been completely banned.

Efforts to restrict or stop using antibiotics in aquaculture | Ministry of Agriculture and Rural Affairs of the People’s Republic of China
The emergence of drug resistance predates the invention of antibiotics.
But the abuse of antibiotics makes it rampant.
Whether in the medical industry or animal husbandry, standardizing the use of antibiotics is to avoid producing drug-resistant bacteria and ensure that after human beings are infected with bacteria, antibiotics can kill bacteria and cure diseases.
However, the emergence of drug-resistant bacteria is not entirely the result of antibiotic use.
A study in 2012 found that there were many kinds of bacteria resistant to potent and advanced antibiotics in Lechuguilla cave, which was isolated from human civilization for nearly 4 million years. Among the 40 antibiotics tested by the researchers, a Bacteroides bacterium with the number LC231 is resistant to 26 of them.

Lechukier Cave | flickr
Another similar example is that in 2013, scientists analyzed a batch of microbial specimens from isolated Indians living in the depths of the Amazon jungle, who had never used antibiotics. The results showed that these microorganisms were resistant not only to natural antibiotics, but also to some synthetic antibiotics.
Therefore, it is actually natural evolution that initially caused bacteria to develop drug resistance.
For a long time, there are many kinds of bacteria in the soil. In order to compete for living space, they have to work hard to find ways to beat their competitors. Some bacteria have evolved the ability to make antibiotics and actively attack other bacteria. For example, the streptomycin we use now comes from streptomyces griseus in the soil. There are also some "Buddha" bacteria that only defend and do not attack. In order to defend their territory, they have evolved a set of mechanisms to resist the attack of foreign antibiotics, which is drug resistance.
Drug resistance is difficult to deal with because it is a defense mechanism that bacteria have evolved for thousands of years.
In the common defense system of bacteria, the outermost layer is the cell wall, which resists foreign attacks like a city wall, and the inner layer is a relatively thin inner membrane, which together protect the interior of bacteria from being destroyed.
When different kinds of antibiotics invade bacteria, they have different strategies:
Classification of attack modes against bacterial antibiotics
(1) by opening holes in the cell wall and cell membrane to launch a large-scale frontal attack, thus killing bacteria, such as polymyxin.
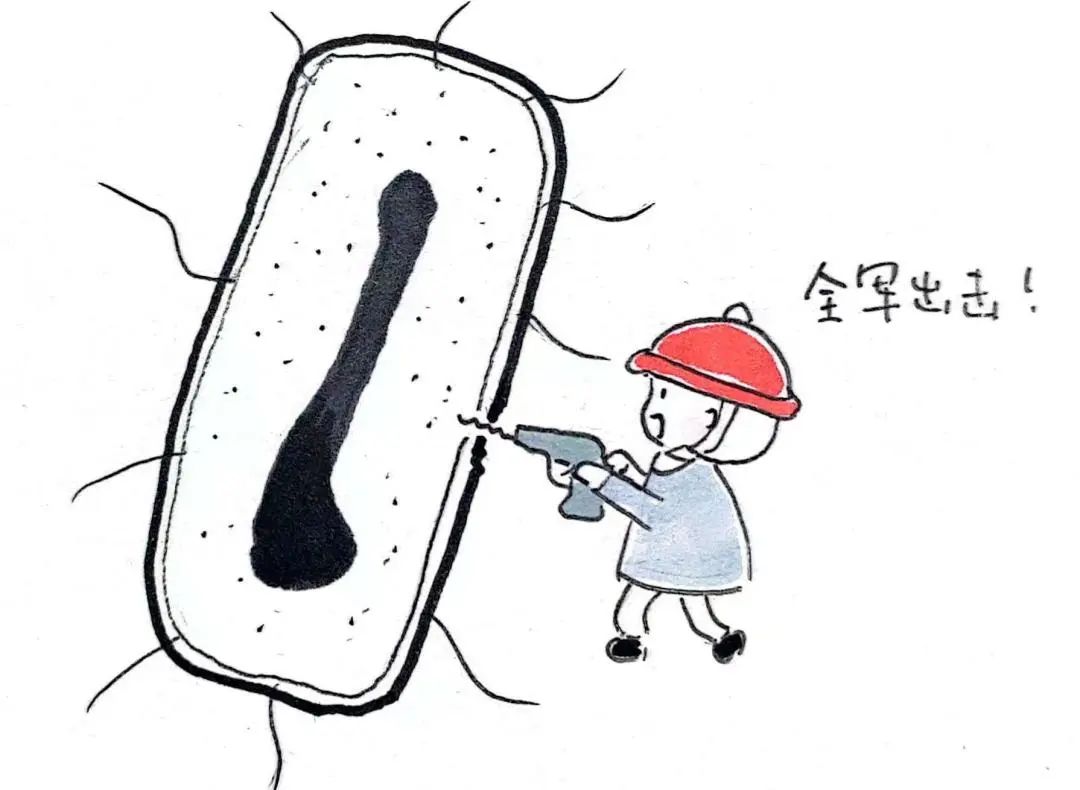
Frontal attack on cell walls and cell membranes | Photo courtesy of the author
(2) to prevent bacteria from building a complete cell wall, so that they lose protection and rupture and die, such as penicillin, cephalosporin, vancomycin, etc.
Prevent bacteria from building cell walls | Photo courtesy of the author
(3) Sneak into bacteria, control the "headquarters" in charge of the synthesis of important substances, interfere with the synthesis of nucleic acids, enzymes, protein, and inhibit bacterial reproduction, such as tetracycline, azithromycin, levofloxacin and sulfonamides.
"Hijacking" the bacterial "headquarters" and interfering with the synthesis of important substances | Photo courtesy of the author
In the process of resistance, bacteria constantly evolve through gene mutation, and some random mutations can just resist the attack of antibiotics, so this part of bacteria survived the battle and passed this mutation on to the next generation, giving future generations the ability to resist antibiotics. There are many forms of these mutations, mainly the following:
Classification of bacterial defense modes
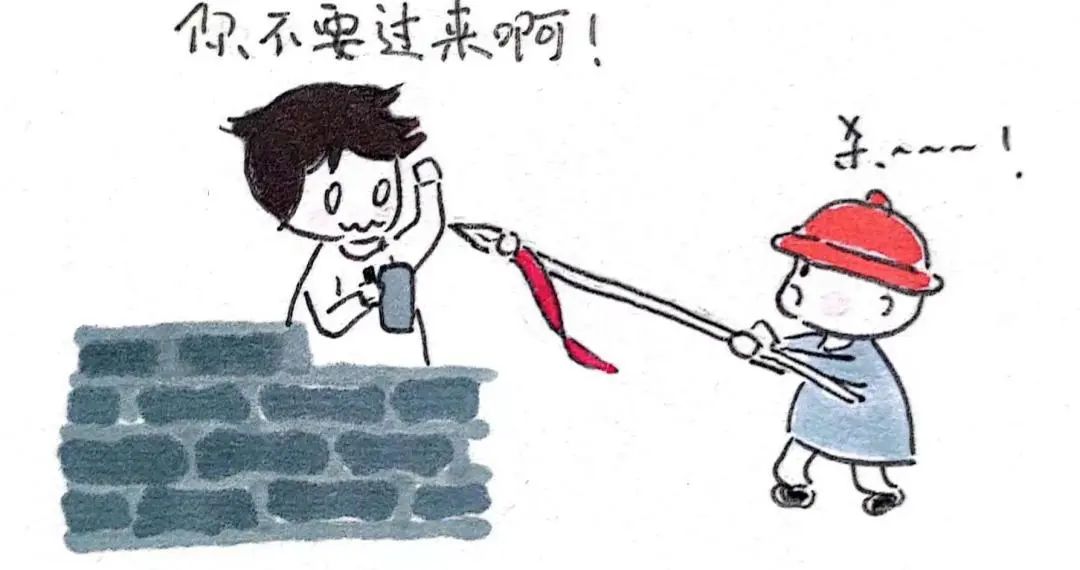
(1) create cell walls that antibiotics can’t recognize, so that they can’t play a role, such as vancomycin-resistant drug-resistant bacteria.
② Shrink the boundary and reduce the permeability of the cell wall, so as to prevent certain kinds of antibiotics from entering the interior, or strictly limit their quantity, and weaken their bactericidal and bacteriostatic effects.
③ Use the efflux pump on the cell membrane to actively pump antibiotics out of the body.
(4) using the cleaving enzyme in bacteria to cut off antibiotic molecules, so that it loses its curative effect. One of the most famous enzymes is called "β-lactamase", which can cut off β -lactam antibiotics such as penicillin, cephalosporin and vancomycin.
⑤ Adding chemical groups to antibiotic molecules makes antibiotics huge and difficult to reach the "headquarters".
⑥ Change the shape or size of the target targeted by antibiotics, so that antibiotics cannot accurately identify and attack. For example, MRSA stopped synthesizing protein, which can be found by the antibiotic methicillin, and instead made another protein with different structure but the same function.
The drug resistance mechanism of drug-resistant bacteria found in Lechukier cave is almost the same as that of the well-known drug-resistant bacteria, and even several more than that previously known by human beings. This is enough to show that the mechanism of bacterial drug resistance is very old, and it may have existed before human activities.
So, what’s the point of restricting the use of antibiotics?
Of course there is.
In natural evolution, for thousands of years, the bacteria that produce antibiotics and the bacteria that resist antibiotics have restricted each other and reached a balanced competition. However, the overuse of antibiotics in the world has led to more and faster evolution of drug resistance of pathogenic bacteria, and drug-resistant bacteria in soil, water, homes and hospitals have flourished and flooded without substantial competition.
Therefore, although the emergence of drug-resistant bacteria is the result of natural evolution, the proliferation of drug-resistant bacteria is indeed caused by antibiotic abuse.
The terrible thing about flooding is that the speed of human research and development of new antibiotics can’t keep up with the speed of drug resistance of pathogenic bacteria. If this continues, there will be more and more infectious diseases without antibiotics, and the number of people who die from drug-resistant bacterial infections will also increase year by year.
The overuse of antibiotics in the world makes pathogenic bacteria evolve drug resistance more and faster | The University of Queensland.
Avoid drug resistance:
You can’t use more or less.
If you want to avoid the proliferation of drug-resistant bacteria and the horror scene at the beginning of the article, there are some ways.
First of all, don’t use antibiotics when they can’t cure the disease.
In the eighties and nineties, for many people, antibiotics were the "standard" for treating cough, fever and diarrhea, and they could be bought at pharmacies at any time without a doctor’s prescription. So, regardless of the cause of discomfort, let’s take an antibiotic first. At that time, in some remote areas, some people even took penicillin as a health care drug and gave it once a year to keep fit.
In many cases, there are no pathogens that can be killed by antibiotics. Antibiotics can only kill or inhibit specific pathogens through the mechanism mentioned above, but besides bacterial or fungal infections, people may get sick by virus infection, allergic reaction, inflammatory diseases, physical or chemical damage, etc. Antibiotics have no therapeutic effect on these situations.
When used in symptomatic cases, antibiotics can not cure diseases, but only increase the risk of drug resistance, without any benefit!
Antibiotics ≠ anti-inflammatory drugs
Many people are used to calling antibiotics "anti-inflammatory drugs". In fact, this statement is wrong, and the confusion of concepts can easily lead to some oolong incidents (click for details).
Strictly speaking, "anti-inflammatory drugs" should be called "anti-inflammatory drugs". Inflammation refers to a defensive response of the human body to stimuli, which is generally manifested as "redness, swelling and heat pain", which can be infectious inflammation caused by pathogens or non-infectious inflammation not caused by pathogens. Anti-inflammatory drugs refer to drugs that can resist these reactions, including steroidal anti-inflammatory drugs (such as glucocorticoid) and non-steroidal anti-inflammatory drugs (such as ibuprofen and acetaminophen).
In a word, the role of antibiotics is to kill or inhibit specific pathogens, and the role of "anti-inflammatory drugs" is to reduce the inflammatory reaction of the body. The roles of the two are different and the applicable diseases are different.
Having said so much about the dangers of antibiotics, should we regard antibiotics as a scourge and ban their use? Of course not! Since penicillin was discovered in 1928, the contribution of antibiotics to human health is beyond doubt.
According to a report in 2016, there are about 214,000 children under the age of 5 who die from drug-resistant infections every year in the world, but the number of people who die because they can’t get antibiotics is twice this number. Obviously, it is impossible to use antibiotics completely at present.
So, some "smart people" found a middle point by themselves: since it is not good to eat more, I will eat less. The doctor asked me to eat for three days if I ate for five days, and the doctor asked me to eat one if I ate two pills …
Please don’t! This is not treating a disease, but making a culture medium for drug-resistant bacteria. Because the pathogens always follow such a golden rule: those who can’t kill me will eventually make me stronger.
When the dosage and use time of antibiotics are not up to standard, all pathogens cannot be killed, and those who survive can easily evolve the best resistance to this antibiotic and pass on the resistance genes-not only to their offspring through reproduction, but also to other bacteria and others.
Therefore, when using antibiotics, we need to find a balance: use it when necessary, and use it correctly and standardly. For ordinary people, the most important thing is to use antibiotics in a symptomatic, adequate and full course of treatment. As for how to do these three things, it is good to use the medicine strictly according to the doctor’s advice.
In addition, for your own health, you should also supervise people around you to take medicine well.
references
[1] Muhammad H.Zaman. History of drug-resistant bacteria [M]. CITIC Publishing Group, 2021.
[2] Matuszewska M, Murray GGR, Ba X, et al. Stable antibiotic resistance and rapid human adaptation in livestock-associated MRSA. Elife. 2022 Jun 28.
[3] Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022 Oct 1.
[4] Bhullar K, Waglechner N, Pawlowski A, et al. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS One. 2012.
[5] Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv. 2015 Apr 3.
[6] Norman A, Hansen LH, S?rensen SJ. Et al. Philos Trans R Soc Lond B Biol Sci. Conjugative plasmids: vessels of the communal gene pool. 2009 Aug 12.
Author: Purple clothes
Read the original text